



Researchers
Enginneers and technicians
.
PhD students
Key words : Olfaction, olfactory receptor, olfactory neurons, central olfactory areas, electrophysiology, neuroanatomy, connectomics, neuroplasticity.
Thematic : Analysis of the olfactory system’s properties and modulation
Research group in detail Open all tabs
Our goal is to investigate how the olfactory system evolves during development and neurogenesis as well as under the influence of the odorant environment and internal metabolic signals. We are using rodents as our main experimental model. We are investigating different levels of modulation, from olfactory receptors to central areas of the brain. We develop molecular, cellular, anatomical and physiological techniques as well as behavioral assays.
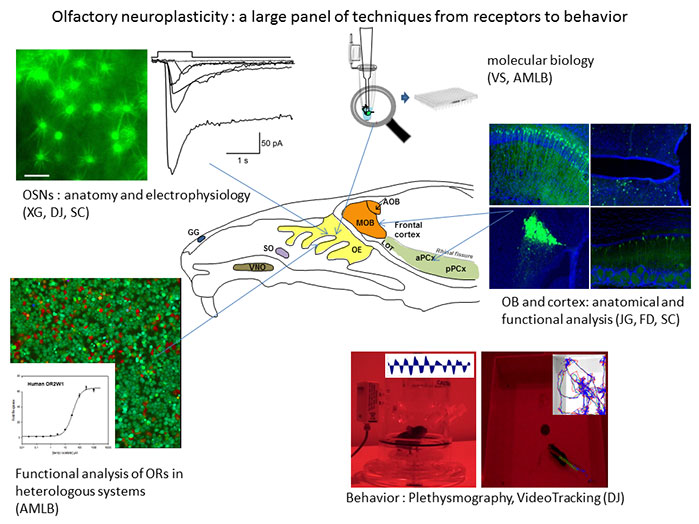
Theme 1 : Functional properties of olfactory receptors and olfactory sensory neurons:
In this topic, we are investigating the membrane properties of olfactory sensory neurons (OSNs) as well as properties of olfactory receptors’ (ORs). We use molecular electrophysiology (patch-clamp) and in vitro approaches. For instance we are focusing on the responses of OSNs and ORs to odorant mixtures. We are using gene-targeted mice in which the fluorescent protein GFP is expressed under the promoter of ORs with a wide spectrum (SR1, olfr15) or with a narrow spectrum (MOR23, M71). Using patch-clamp, we can characterize the responses of these neurons to single odorant molecules or odorant molecules in a mix. We also express ORs in heterologous systems in order to evaluate their intrinsic properties through functional tests.
We are also interested in the functional properties and role of olfactory neurons during development of the olfactory system. This project is developed in collaboration with the laboratory of Peter Mombaerts, Max Planck Research Unit for Neurogenetics, Frankfurt, Germany. Our aim is to better understand how olfactory receptors are involved in the addressing of axons during neurogenesis. To this end, using gene-targeted mice, we replace the expression of the olfactory receptor with another G protein-coupled receptor (GPCR), such as beta-adrenergic receptor. In Dijon, we then characterize the membrane properties of these OSNs expressing the other type of GPCR using patch clamp (Omura et al, 2014).
Theme 2 : Odorant induced plasticity in olfactory neurons
Our goal is to understand the consequences of the odorant environment on the properties of olfactory neurons. After exposure to odorant molecules we measure the anatomical, molecular and physiological effects on neuronal populations labeled with GFP. We observed that during post-natal development, exposure to their cognate ligand induced plasticity on certain populations of neurons (Cadiou, Aoude et al, 2014). We are currently investigating the consequences on the properties of OSNs of the odorant exposure during adulthood or during embryonic development. We also investigated the anatomical and molecular effects of sensory deprivation during the postnatal development. We observed that unilateral naris closure induced different effects on different OSNs’ populations (Molinas et al, 2016).
Theme 3 : Plasticity of the olfactory system induced by homeostatic changes: effects of nutritional status and diet.
The objective of this project is to evaluate the effects of diet on the physiology of the olfactory system (peripheral and central levels) and olfactory behaviors. We use two types of diet inducing metabolic disorders: a high fructose diet rapidly inducing diabetes, and a high fat high sugar inducing both diabetes and obesity. We assess the impact of these diets i) on the physiology of the olfactory epithelium using electrophysiology (EOG and patch-clamp), molecular biology and immunohistochemistry; ii) on the status of the OSNs’ projections into the olfactory bulb by immunohistochemistry and on the functional properties of the olfactory bulb using c-fos labelling; iii) the olfactory behavior by basic tests (habituation / dishabituation, buried food, plethysmography).
Theme 4 : Neural structures involved in olfactory, hedonic and feeding behaviors: connections, plasticity and behavioral impact.
Olfaction plays a key role in the hedonic component of feeding behavior. On one hand, some odors, considered as highly appetitive, trigger food intake; on the other hand, through specific sensory satiation, there is a gradual reduction of food-elicited pleasure during food consumption. The neural correlates of these phenomena remain unknown. To address this challenge, it is required to describe the connectome between the central structures integrating olfactory, homeostatic (hypothalamus ...) and reward (ventral tegmental area ...) informations. We use stereotactic injections of retrograde tracers such as polysynaptic virus (PRV) and monosynaptic cholera toxin (CTb). Double-immunocytochemical characterization of the retrogradely labeled neurons is also performed. The functional significance of the identified circuits might be investigated using behavioural paradigms.
Merle, L., Person, O., Bonnet, P., Grégoire, S., Soubeyre, V., Grosmaitre, X. and Jarriault, D. (2019). Maternal high fat high sugar diet disrupts olfactory behavior but not mucosa sensitivity in the offspring. Psychoneuroendocrinology 104: 249-258. doi: 10.1016/j.psyneuen.2019.02.005.
Nocera, S., Simon, A., Fiquet, O., Chen, Y., Gascuel, J., Datiche, F., Schneider, N., Epelbaum, J. and Viollet, C. (2019). Somatostatin serves a modulatory role in the mouse olfactory bulb: Neuroanatomical and behavioral evidence. Frontiers in Behavioral Neuroscience 13(61): doi: 10.3389/fnbeh.2019.00061
Schneider, N. Y., Chaudy, S., Epstein, A. L., Viollet, C., Benani, A., Pénicaud, L., Grosmaître, X., Datiche, F. and Gascuel, J. (2019). Centrifugal projections to the main olfactory bulb revealed by trans-synaptic retrograde tracing in mice. Journal of Comparative Neurology. doi: 10.1002/cne.24846
Le Bon, A. M., Deprêtre, N., Sibille, E., Cabaret, S., Grégoire, S., Soubeyre, V., Masson, E., Acar, N., Bretillon, L., Grosmaitre, X. and Berdeaux, O. (2018). Comprehensive study of rodent olfactory tissue lipid composition. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA) 131: 32-43. doi: 10.1016/j.plefa.2018.03.008
Belloir, C., Miller-Leseigneur, M.-L., Neiers, F., Briand, L. and Le Bon, A.-M. (2017). Biophysical and functional characterization of the human olfactory receptor OR1A1 expressed in a mammalian inducible cell line. Protein Expression and Purification 129: 31-43. doi:10.1016/j.pep.2016.09.006
Francois, A., Bombail, V., Jarriault, D., Acquistapace, A., Grebert, D., Grosmaitre, X. and Meunier, N. (2017). Daily oscillation of odorant detection in rat olfactory epithelium. European Journal of Neuroscience 45(12): 1613-1622. doi: 10.1111/ejn.13600
El Mountassir, F., Belloir, C., Briand, L., Thomas Danguin, T. and Le Bon, A.-M. (2016). Encoding odorant mixtures by human olfactory receptors. Flavour and Fragrance Journal 31(5): 400-407. doi: 10.1002/ffj.3331
Molinas, A., Aoude, I., Soubeyre, V., Tazir, B., Cadiou, H. and Grosmaitre, X. (2016). Anatomical and molecular consequences of Unilateral Naris Closure on two populations of olfactory sensory neurons expressing defined odorant receptors. Neuroscience Letters 626: 42-47. doi: 10.1016/j.neulet.2016.05.027
Rivière, S., Soubeyre, V., Jarriault, D., Molinas, A., Léger-Charnay, E., Desmoulins, L., Grebert, D., Meunier, N. and Grosmaitre, X. (2016). High fructose diet inducing diabetes rapidly impacts olfactory epithelium and behavior in mice. Scientific Reports 6: 34011. doi: 10.1038/srep34011
Tazir, B., Khan, M., Mombaerts, P. and Grosmaitre, X. (2016). The extremely broad odorant response profile of mouse olfactory sensory neurons expressing the odorant receptor MOR256-17 includes trace amine-associated receptor ligands. European Journal of Neuroscience 43(5): 608-617. doi: 10.1111/ejn.13153
Connelly, T., Yu, Y., Grosmaitre, X., Wang, J., Santarelli, L. C., Savigner, A., Qiao, X., Wang, Z., Storm, D. R. and Ma, M. (2015). G protein-coupled odorant receptors underlie mechanosensitivity in mammalian olfactory sensory neurons. Proceedings of the National Academy of Sciences of the United States of America 112(2): 590-595. doi: 10.1073/pnas.1418515112
Jarriault, D. and Grosmaitre, X. (2015). Perforated patch-clamp recording of mouse olfactory sensory neurons in intact neuroepithelium: functional analysis of neurons expressing an identified odorant receptor. Journal of Visualized Experiments: JoVE 101: e52652. doi: 10.3791/52652

